Introduction
Solid tumors present an ongoing challenge in immunotherapy, owing largely to the complexities of the tumor microenvironment (TME). While immunotherapies have drastically improved outcomes for many patients with these malignancies, not all solid tumors are equal; even best-in-class immunotherapies fail to induce durable responses in the majority of patients with solid tumors.
But why is this the case? And what can we do about it? In this article we’re delving into the science of so-called ‘cold’ tumors: those tumors that don’t respond to traditional immunotherapy. We’ll explore the concepts of ‘hot’ and ‘cold’ tumors, the major factors influencing their classification, and emerging strategies to turn ‘cold tumors’ into ‘hot’ tumors.
Understanding the Cold Tumor Microenvironment
There are actually several different categories of tumor, based on what is known as their ‘immunophenotype’ or immunoscore: the relative presence or absence of cytotoxic lymphocytes, like CD8+ T cells and natural killer (NK) cells, in the TME.
These immunophenotypes exist on a short spectrum ranging from ‘cold’ to ‘hot’, and they determine the likeliness of a tumor responding to immunotherapy. Let’s explore cold tumors, hot tumors, the differences between them, and why cold tumors are such a problem in the field of cancer immunotherapy.
What is a cold tumor?
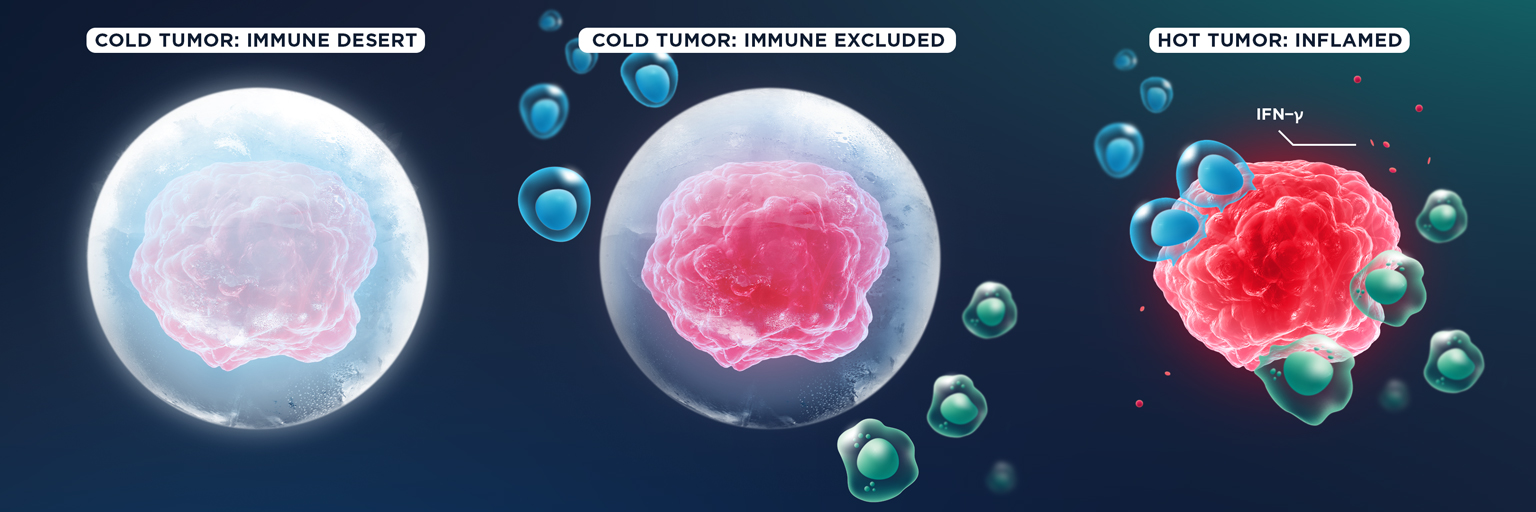
A cold tumor is one in which there is limited or no immune activity – they are, essentially, resistant to the immune system. Cold tumors are characterised by several key features: an absence of tumor-infiltrating lymphocytes (TILs), low expression of major histocompatibility complex (MHC) class 1 and immune checkpoints like programmed death-ligand 1 (PD-L1), low tumor mutational burden (TMB), and a lack of prior anti-tumor immune response.
The term ‘cold’ shouldn’t be taken in a literal sense. The name means that they are immunologically cold – there is no inflammation in the tumor that would allow the immune system to combat them naturally. If we think of the tumor as a battlefield, there are no soldiers – those CD8+ T and NK cells we mentioned earlier – on the battlefield of a cold tumor to fight the cancer cells.
The term cold tumor covers two subcategories: immune-excluded tumors and immune-desert tumors. In the former group, cytotoxic lymphocytes like CD8+ T cells may be present in the periphery of the tumor, but they are unable to infiltrate. In the latter, immune cells are nowhere to be seen.
So why can’t cytotoxic lymphocytes infiltrate cold tumors? There are a few reasons. Cold tumors have a low TMB, which means they have significantly fewer new tumor antigens. They may also have low expression of MHC, which is the machinery the cells use to present tumor antigens on its surface.
Because antigens are crucial for T cell priming and recognition of cancer cells, these factors prevent CD8+ T cells from doing their job. Another major contributor is the fact that cold tumors typically lack the pro-inflammatory cytokines that help recruit these soldiers to the battlefield.
Cold tumors often have more immunosuppressive cells, such as tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) and regulatory CD4+ T cells (Tregs). These cells have evolved multiple pathways to negatively regulate CD8+ T cell fitness and function, so they can impair CD8+ T cell persistence and activity in cold tumors.
Cold tumor vs. hot tumor: what’s the difference?
You guessed it – hot tumors are basically the opposite of a cold tumor. Hot tumors have an inflamed phenotype and are characterized by robust production of pro-inflammatory cytokines like interferon gamma (IFN-γ), an abundance of TILs within the TME, overexpression of PD-L1, genomic instability and/or a high TMB, and evidence of prior antitumor immune responses.
Hot tumors are most likely to respond to immunotherapies, like immune checkpoint inhibitors (ICI) or immune checkpoint blockade (ICB), while cold tumors are very unlikely to respond.
List of hot and cold tumors
You might be wondering which tumors are hot and which are cold. Unfortunately, producing a list of hot and cold tumors is not quite that simple. As we’ve already discussed, the factors that contribute to the TME are complicated, and while some types of tumors are generally hot and consistently respond better to ICI than others, there’s no golden rule; many patients will have cold tumors, regardless of the type of cancer they have.
Some types of cancer are generally more prone to developing as hot tumors, including melanoma, non small cell lung cancer (NSCLC), renal cell carcinoma and mismatch-repair deficient colorectal cancer (MMRd-CCR). Others, such as glioblastoma, prostate cancer, and pancreatic cancer, are more likely to be cold tumors.
Why are cold tumors a problem?
Cold tumors present a key challenge in cancer treatment because of their immune resistance and general lack of visibility to the immune system. As such, there is little chance that even the best immunotherapies available can treat them successfully.
Let’s take anti-PD-L1 therapy as an example. PD-L1 is overexpressed on the cells of a hot tumor thanks to the secretion of IFN-γ, and binds to a receptor expressed on T cells called PD-1. This binding prevents the T cells from killing cancer cells, allowing the tumor to continue growing. If we think of T cells as a car speeding towards the tumor, then the interaction between PD-L1 and PD-1 is like someone has slammed their foot on the brakes.
However, immunologists are able to exploit this pathway by using ICIs – molecules that can block PD-L1, preventing it from binding to the PD-1 receptor on T cells. They can also achieve the same outcome by blocking PD-1. This type of therapy effectively lifts that foot off the brakes of the car, allowing T cells to do their job.
This type of therapy is known as anti-PD-1/PD-L1 therapy, or PD-1/PD-L1 blockade, and is considered one of the most effective immunotherapies available today. However, if a patient has a cold tumor, there is typically low expression of PD-L1 to begin with, and that means the tumor won’t respond to PD-L1 inhibitors.
Instead, a cold tumor is typically treated with traditional drugs such as chemotherapy and radiation therapy. In some relevant cases, a cold tumor may also be treated with targeted inhibitors against oncogenic mutant proteins.
Immunotherapy: How Do We Heat Up a Cold Tumor?
One of the aims of emerging immunotherapy approaches is to ‘heat up’ immunologically cold tumors: in other words, to increase their visibility to the immune system, increase TILs, and increase the sensitivity of the tumor to ICI. Achieving this goal would extend the durable benefits of immunotherapy to a far greater number of solid tumor patients.
However, turning a cold tumor into a hot tumor requires a deep understanding of the immune system and cancer biology. Let’s take a look at the factors that keep tumors cold, how we can heat up cold tumors, and the strategies we’re using here at oNKo-innate to tackle the cold tumor problem.
Understanding T cell homing to tumors: the role of CXCL9
Understanding why cold tumors lack TILs is critical if we aim to turn them into hot tumors. As it happens, one of oNKo-innate’s very own senior scientists, Dr. Imran House, played a key role in solving this mystery.
If you read our last blog on cytokines, you might remember we mentioned some specialist molecules called chemokines. Chemokines have a very important job, trafficking immune cells around the body. In the context of cancer, chemokines direct cytotoxic lymphocytes to tumor draining lymph nodes, where they are primed by antigen presenting cells (APCs). Lymphocytes are then directed back to the tumor so they can infiltrate the TME and kill cancer cells.
T cells express a receptor called CXCR3, which binds to the chemokines CXCL9, CXCL10, and CXCL11. Primarily produced by anti-tumor macrophages known as M1 macrophages, CXCL9 in particular is now known to be crucial for activating T cells and for recruiting them to tumors so they can infiltrate the TME.
High levels of CXCL9 and CXCL10 are strongly correlated with IFN-γ production and improved clinical outcomes in patients treated with anti-PD-1 therapy. Expression of CXCL9 can also be used as a surrogate marker to determine if a tumor is cold and predict its response to immunotherapy.
The importance of CXCL9 in modulating the immune response to tumors means it can also be exploited to enhance responses to existing immunotherapies, potentially heating up cold tumors. For example, a molecule that can rewire macrophages and nudge them toward an anti-tumor M1 phenotype – causing them to produce more CXCL9 – could be used as a combination immunotherapy with anti-PD1 or PD-L1 therapy and improve overall treatment outcomes. For more information on cytokine therapies, check out our article on this topic below.
Turbocharging cytotoxic lymphocytes with cytokines
Here at oNKo-innate, we’re leading the charge against solid tumors and we’re committed to the goal of turning cold tumors hot using novel immunotherapies. If we go back to the earlier analogy and think of T cells as a car, PD-L1/PD-1 blockade therapy is like taking the brakes off the car. But as we discussed, cold tumors don’t respond to this approach. So how can we get that car to move forward? By turbocharging it.
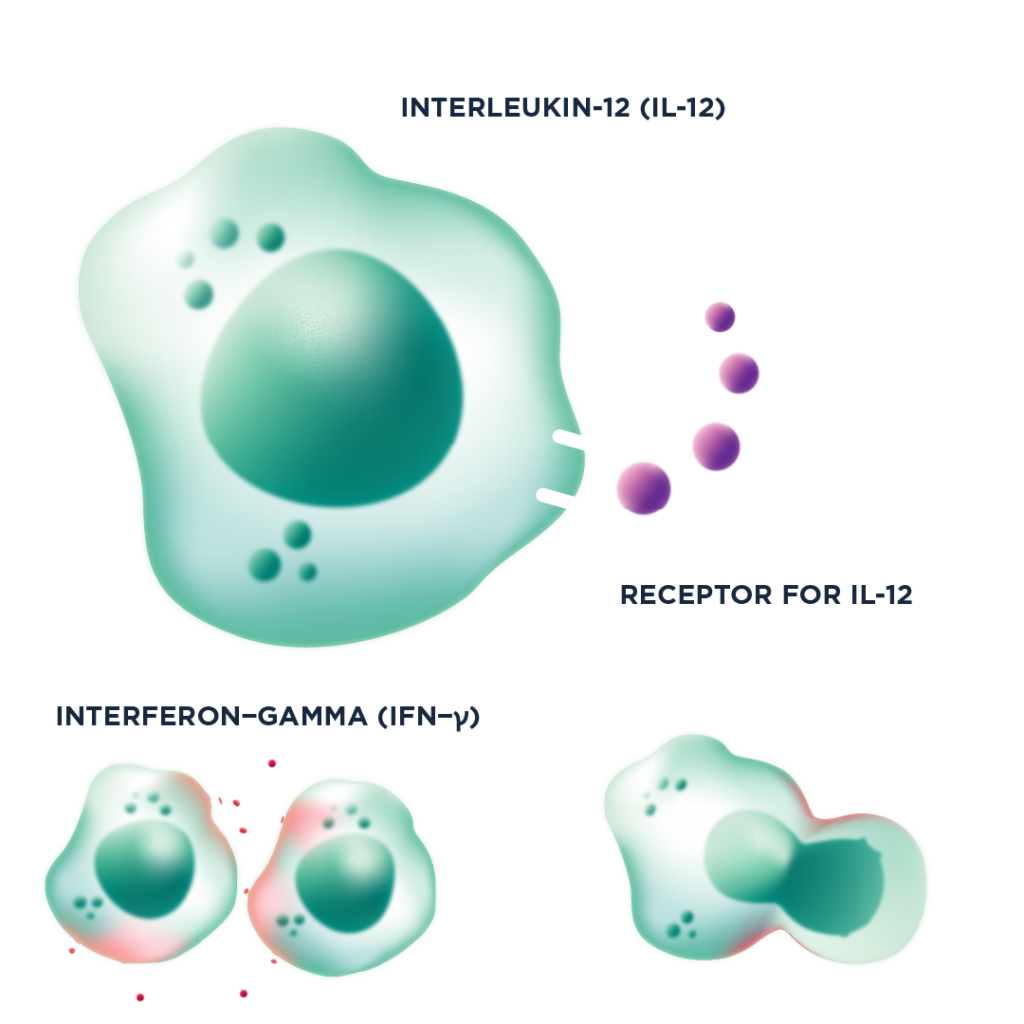
While PD-1/PD-L1 blockade can’t heat up a tumor, cytokines absolutely can. Pro-inflammatory cytokines like IL-12 activate TILs – including CD8+ T cells and NK cells – causing them to proliferate and dialling up their anti-tumor function, essentially acting like a turbocharger for the car. For example, IL-12 is a potent inducer of IFN-γ production, which in turn upregulates MHC-I. This helps prime CD8+ T cells in the lymph nodes and makes tumors more visible to those CD8+ T cells. The elevated production of IFN-γ also results in an increase in PD-1 expression, meaning that IL-12 can produce synergistic effects when used in concert with anti-PD-1/PD-L1 therapies.
oNKo-001 cytokine therapy
oNKo-innate has two current pipeline assets that take different approaches to cytokine therapy. oNKo-001 is an engineered, reduced potency IL-12 fusion protein that aims to defeat solid tumors, particularly those that don’t respond to traditional immunotherapies like PD-1/PD-L1 blockade.
This therapy induces a potent anti-tumor response in CD8+ T and NK cells, but lacks the systemic toxicity associated with wild-type IL-12. It also has a much longer half-life in vivo, reducing the need for regular dosing. When it comes to IL-12 therapies, we believe in the “low and slow” approach to keep the anti-tumor activity safe, but durable.
oNKo-037: A cytokine checkpoint inhibitor
As an alternative approach to oNKo-001, our oNKo-037 therapy is an IL-15 cytokine checkpoint inhibitor. Because IL-15 helps CD8+ T cells and NK cells work better within the TME, it’s a strong player in regulating anti-tumor immunity.
The problem is that solid tumors often do not have optimal levels of endogenous IL-15. oNKo-037 works to increase the sensitivity of CD8+ T cells and NK cells to these low levels of IL-15 so that they can still do their job, even in the most hostile environments.
Optimal CD8+ T cell function requires three important signals. Signal 1 is the T cell receptor (TCR), and signal 2 is the T cell co-receptors. Anti-PD1 therapy predominantly improves these first two signals. However, IL-15 is a crucial driver of signal 3, and oNKo-037 predominantly improves this signal.
This is why the combination of anti-PD1 therapy and oNKo-037 has the potential to fully restore CD8+ T cell function in a variety of solid cancers. We believe that dialling up signal 3 will increase the number of patients that respond to ICI therapy, which targets signal 1 & 2.
The Final Frontier of Solid Tumor Therapy
The development of a therapy that could heat up cold tumors would be a major breakthrough in the field of immunotherapy, benefiting many patients with solid tumors. While we’ve placed an emphasis on targeting immune cells to heat up cold tumors in this article, there are also several tumor-intrinsic drug targets that have been identified. The inhibition of these targets results in increased immunogenicity – the visibility of tumor cells to the immune system.
Several of these targets work by negatively regulating factors such as proinflammatory chemokine production, stress-responses and immunogenic cell death pathways, and antigen processing or presentation machinery. Inhibition of these targets in solid tumors are currently being investigated in combination with ICI, as an alternative approach to turn up the heat in cold tumors.
As cancer researchers work towards the goal of heating up cold tumors, multiple strategies are being developed and trialled that increase the infiltration, activity and resistance of immune cells to suppression within cold tumors. Ultimately, the best approach is likely to vary across the different tumor types, based on TMB and oncogenic mutations, as well as disease burden, stage, and location.
Improving immunotherapy response rates in hard-to-treat solid tumors is a goal we share with the wider community, from biotech to the pharmaceutical industry, and researchers from both clinical and academic perspectives. We believe that taking multiple approaches to this goal is the best way to get the desired results and transform the lives of patients.
We hope this article helped you get your head around the concept of cold tumors and why they’re such a big obstacle in the field of cancer immunotherapy. If you want to learn more about immuno-oncology, novel cancer therapies, and the complexities of the tumor microenvironment, be sure to check out the rest of our Learning Centre, because we’ll be posting more articles soon. You can also follow us on X and LinkedIn for regular updates.